|
Until
now, freezing and testing of
PBMC has required the use of
serum.
At isolation, PBMC need to
make a critical transition from
ideal in vivo conditions
into the artificial tissue culture
environment. Carefully selected,
naturally rich serum has been
essential for this transition.
Serum contains a plethora of
bioactive molecules whose variable
concentrations makes every serum
batch unique. Careful testing
of lots has been time-consuming
yet necessary, and the limited
size of each lot makes this
testing a repeated inconvenience.
In addition, working with serum
is expensive. At the approximate
cost of $1.000/L for human AB
serum, complete RPMI medium
made up in the lab is costly,
and the need for sterile filtering
each time adds to the expense.
The cost is further increased
by the need to purchase and
store larger lots of a serum
batch (Table 1).
 From the good
scientific/laboratory practice
perspective, even an "ideal"
serum batch is a unique reagent
affecting T cell performance
in vitro . The need
to work with serum has been
a major obstacle to reproducible
and standardized ex vivo
experimentation.
CTL
has introduced media for serum-free
work with PBMC
Unlike for tumor cell lines
that have adapted to tissue
culture conditions, ideally
performing serum-free media
for ex vivo human T
cell work were not available.
CTL's scientist have formulated
a media portfolio that permits
to perform serum free all steps
of the ex vivo work
with PBMC: the freezing, washing,
and the testing of these cells.
These media are:
- Convenient: avoiding the
repeated effort for selecting
"good" serum batches.
- Standardized: the same "serum
substitute" can be used over
and over, in the same and
different laboratories.
- Cost effective: CTL's serum
free media have been priced
not to exceed the cost of
traditional serum-containing
media.
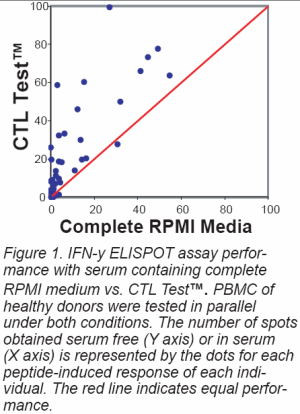
- Of ultimate importance:
CTL's serum-free media outperforms
even the "best" serum batches
(Figure 1).
|
|
 |
| |
CTL's
serum-free portfolio consists
of a PBMC freezing medium, washing
medium, and testing medium.
CTL
freezing media: CTL-Cryo?/span>
When
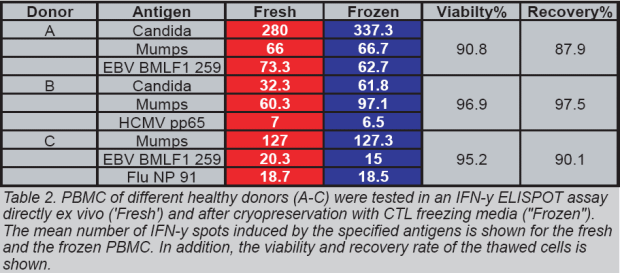
frozen according to our protocols,
PBMC maintain their full functionality
in ELISPOT assays (Table 2).
The ability to freeze PBMC can
be of major benefit, e.g. for
clinical trials. First, instead
of having to test the individual
PBMC samples as they are obtained,
they can be run economically
in large batches. Second, samples
from the same individuals obtained
at different time points, and
samples of individuals belonging
to different groups can be run
side-by-side, under identical
conditions, avoiding inter-assay
variability. Third, assay results
can be reproduced, if necessary,
on different aliquots from the
same blood draw. Lastly, cryopreservation
allows screening in a first
assay for overall reactivity
and then retesting of the
sample to closer define the
fine specificity, CD4/CD8 lineage,
affinity, and cytokine profile
of the specific T cells.
CTL
washing media: CTL Wash?/span>
PBMC
need to be washed after the
Ficoll isolation before they
are tested as fresh cells or
are frozen. Washing is also
required afterthawing the PBMC.
Washing media typically contains
serum because washing in saline
or PBS alone reduces the PBMC's
performance, and because the
presence of proteins prevents
cell clumping. However, even
brief exposure of the PBMC to
nonstandardized (e.g. mitogenic
or toxic) serum will fundamentally
affect the PBMC's performance.
CTL's new serum-free washing
media offers a standardized,
convenient and cost-efficient
alternative. |
|
CTL
testing media: CTL Test?/span>
When
PBMC are used for immune monitoring,
few antigen-specific T cells
need to be detected amongst
hundreds of thousands non relevant
PBMC. Low background and strong
signal resulting in maximal
assay resolution are critical.
Test media for such T cell assays
have traditionally contained
human AB serum that, frequently
cause high background, or low
signal. CTL's new serum-free
testing media consistently provide
lower noise and higher signal
in such assays that even the
"best" serum batches (Figure
1). They are standardized, convenient
and cost-efficient.
 |
